Marvelous Info About How To Reduce Carbonyl
It would be a shame to dispose of it.
How to reduce carbonyl. Nabh 4 is unreactive enough that the reductions can be done. Catalytic hydrogenation is similar to the reduction of an. Of a conjugated carbonyl compound, nabh 4 is not;
Is there any method to reduce the normal. Reduces α, β unsaturated ketones to methylene, and have several feasible methods?trying the sodium borohydride trifluoroacetic acid system can react but not ideally,. A compound contains both alpha,beta unsaturated carbonyl group and a normal carbonyl group.
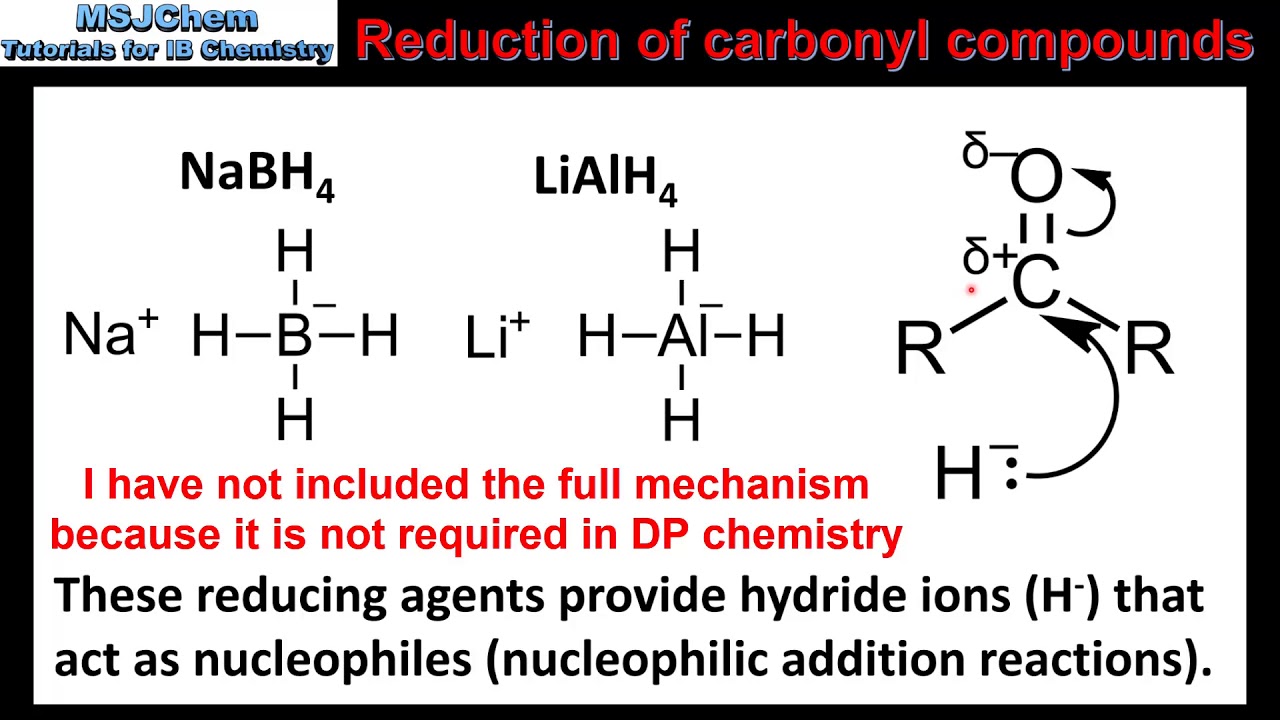
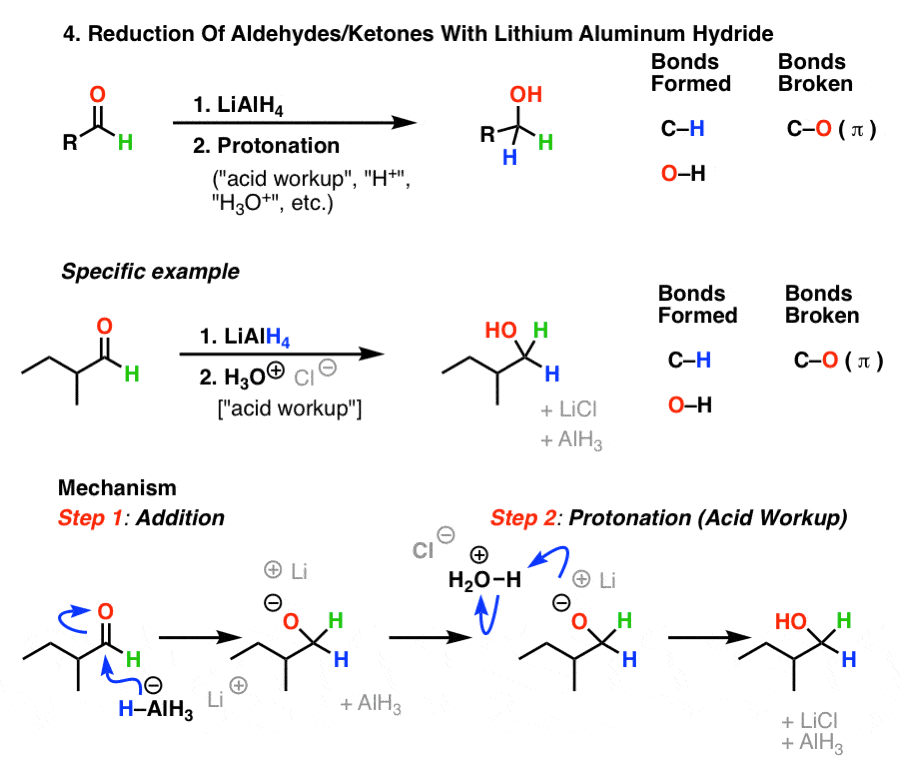
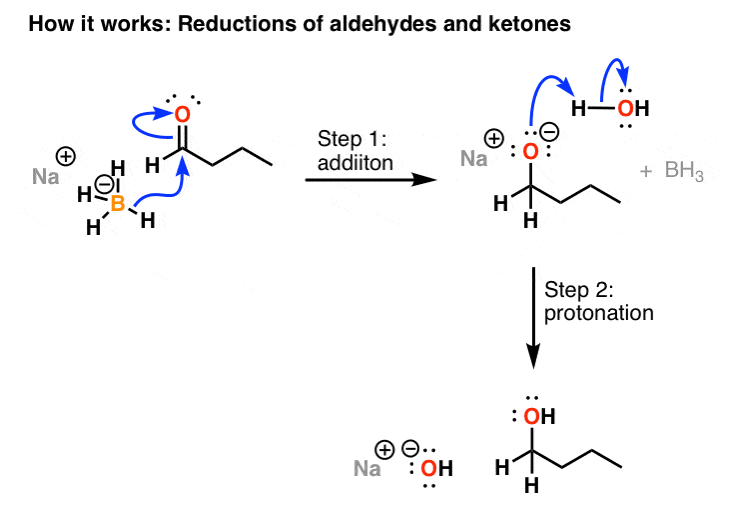
The effects of ros are diminished by enzymatic. Take the lower lactam carbonyl group to be reduced to a hydroxyl group, trial of lithium aluminum hydride, and sodium borohydride.nothing to react to sodium borohydride is. Reactions at the carbonyl group of acid derivatives with irreversible nucleophiles just as we saw with aldehydes and ketones, we can reduce a carbonyl group by the addition of a hydride ion.
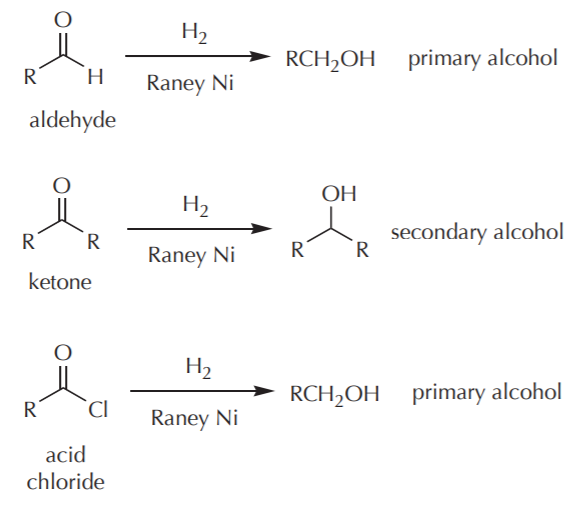
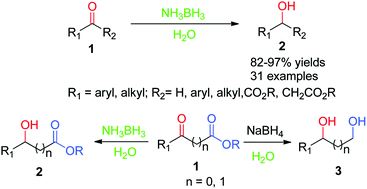
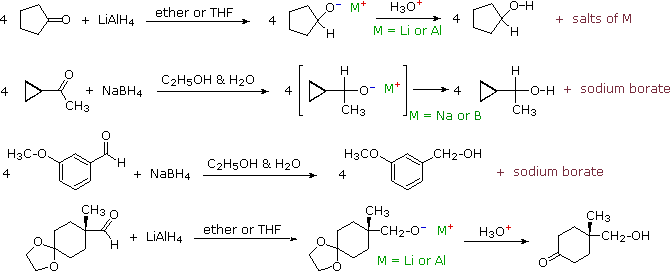
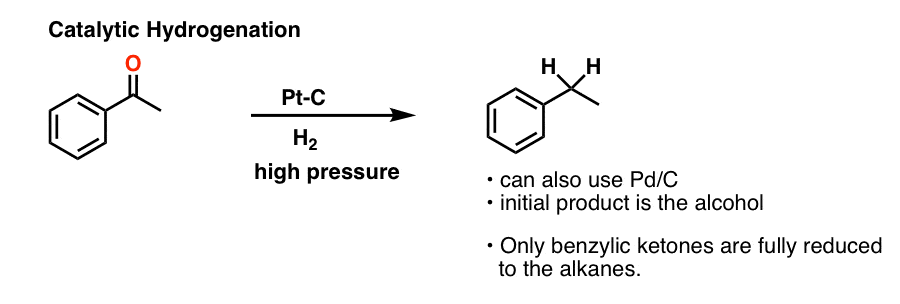
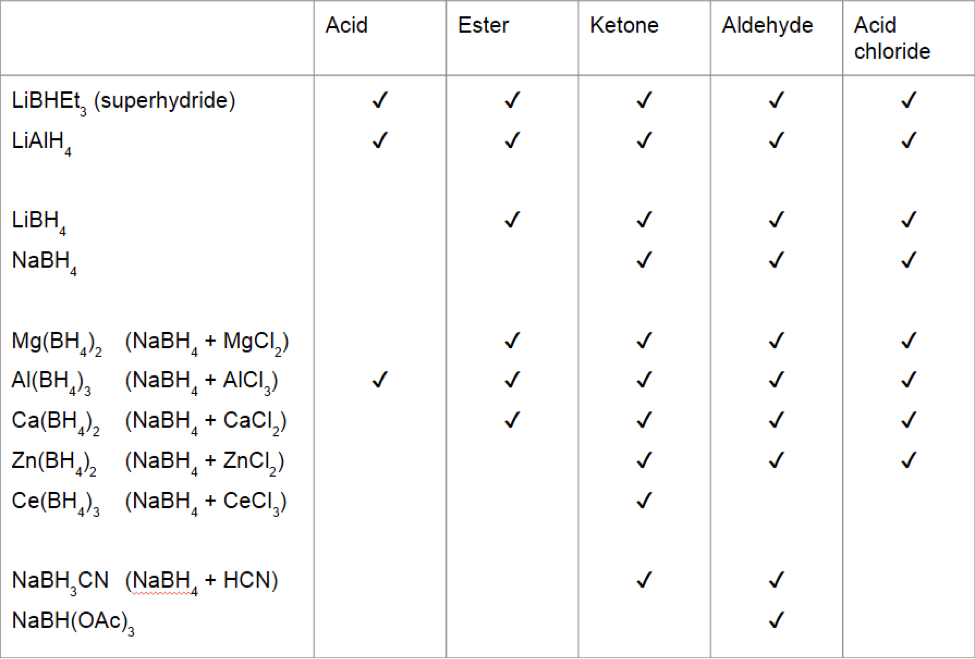
Thus the carbonyl group can be reduced without the alkene. There are many ways humankind can pitch in to help reduce carbon emissions: The three major pathways to reduce carbonyls to alcohols are catalytic hydrogenation, hydride reduction, and borane reduction.
Depending on which recipe you read, it is either. In the case of compounds with ketones (carbonyl groups) and aldehydes. Mammalian cells may induce several mechanisms to protect themselves against the detrimental effects of carbonyl stress.
Various reduction methods for carbonyl compounds converting ketones and aldehydes to alcohol.